What is RF Value?
The Rf value (retardation factor/Retention Factor) is an important parameter in chromatography that represents the distance traveled by a particular compound relative to the distance traveled by the solvent front.
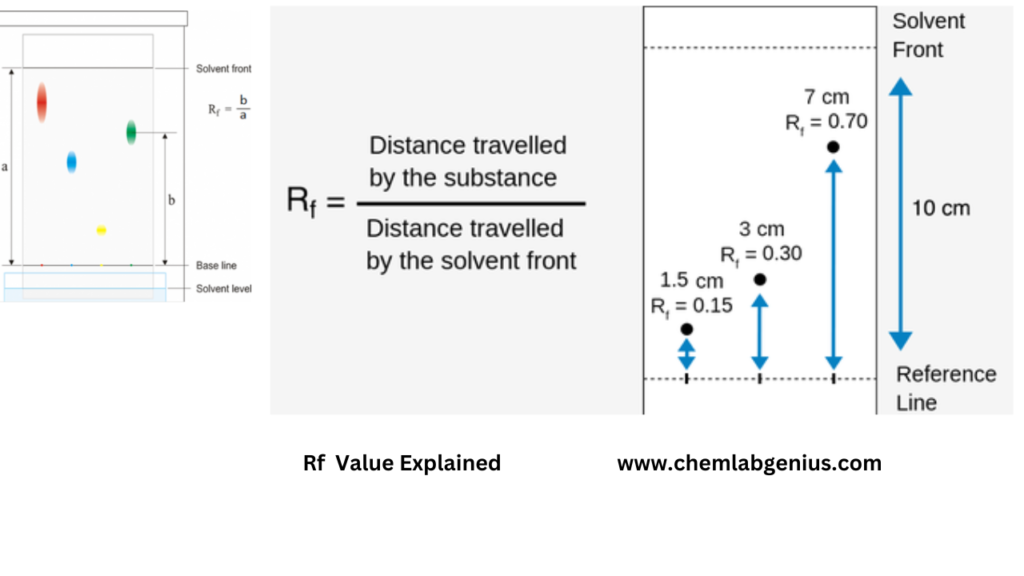
The Rf value is a dimensionless number that ranges from 0 to 1. It is calculated by dividing the distance traveled by the compound by the distance traveled by the solvent front. The resulting Rf value represents the relative mobility of the compound in the chromatogram.
Calculation of RF Value
The Rf value can be calculated using the following formula:
Rf = distance traveled by the compound / distance traveled by the solvent front
The distance traveled by the compound is measured from the origin to the center of the spot, while the distance traveled by the solvent front is measured from the origin to the solvent front.
Why Do We Need the RF Value?
The Rf value is an essential parameter in chromatography because it allows for the identification and analysis of individual components in a mixture. By comparing the Rf values of an unknown sample to those of known compounds, scientists can determine the identity of the unknown compound.
The Rf value also provides information on the polarity of the compound. Polar compounds tend to have a lower Rf value, while nonpolar compounds tend to have a higher Rf value. This information can be useful in determining the chemical properties of a compound.
Factors Affecting Rf Values
The Rf value is influenced by various factors, including:
- The nature of the stationary phase: Different stationary phases have different affinities for compounds, which can affect their mobility in the chromatogram.
- The composition of the mobile phase: The composition of the mobile phase can affect the solubility and polarity of the compounds, which can in turn affect their mobility.
- The temperature and pressure of the system: Changes in temperature and pressure can affect the viscosity and density of the solvent, which can affect the mobility of the compounds.
- The size and shape of the spot: The size and shape of the spot containing the compound can affect its Rf value.
Further Insights on RF Value
- The Rf value is dependent on the type of chromatography technique being used, such as thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), or gas chromatography (GC).
- The Rf value can be affected by the pH of the solvent system. Compounds with ionizable groups may exhibit different Rf values at different pH values.
- The Rf value can be used to determine the purity of a compound. A pure compound will have a single spot with a consistent Rf value, while impure compounds will have multiple spots with varying Rf values.
- The Rf value can also be used to estimate the molecular weight of a compound. Larger molecules tend to have a lower Rf value, while smaller molecules tend to have a higher Rf value.
- In some cases, the Rf value may not be the best parameter to use for compound identification. Other parameters such as retention time, peak area, and peak height may be more appropriate for certain types of chromatography.
FAQs Related to RF value
- What is a good Rf value?
A good Rf value is one that is consistent with the known Rf values of the compound under the same experimental conditions. However, there is no universal “good” Rf value, as it can vary depending on the system and the compound being analyzed.
- Can two compounds have the same Rf value?
Yes, two compounds can have the same Rf value if they have similar chemical properties and interact similarly with the stationary and mobile phases in the chromatography system.
- Can the Rf value be greater than 1?
No, the Rf value cannot be greater than 1. If the compound travels farther than the solvent front, it may have been over-spotted or over-applied, or the solvent system may not be appropriate for the compound.
